Your Location:Home > Products > Organic Chemistry > Benzylmagnesium chloride

CasNo: 6921-34-2
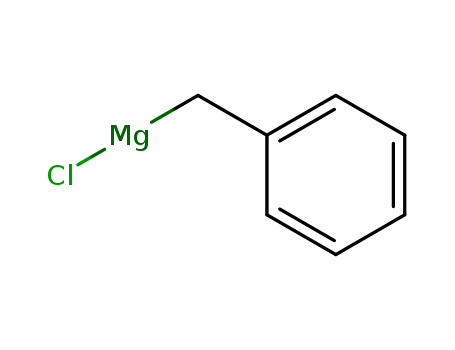
MF: C7H7ClMg
Appearance: clear green-brown to brown-purple solution
|
Research |
Grignard reagents are organometallic compounds discovered by a French chemist, Victor Grignard in 1900. These compounds can be obtained as a formula “RMgX” by the reaction of halogenated hydrocarbons with magnesium metal in anhydrous solvents such as diethyl ether,1) and have been widely used in organic synthesis.Grignard reagents are usually used as solutions, and they are typically grayish white ~ light brown clear solutions. They generally exist in an equilibrium between alkyl magnesium halides (RMgX) and dialkyl magnesium (R2Mg), which form equilibrium mixtures with complicated compositions (called “Schlenk equilibrium”).2) Furthermore, some of these species may cause precipitation due to their lower solubility. However, there is no problem in actual use.In the case of precipitation being formed:Please use it after gently shaking the bottle to fully disperse the suspension (If the amount of precipitation is very small, you can use the top clear layer of the suspension). Especially in cold conditions such as in winter, a large amount of precipitate may frequently form. In this case, please use it after crushing the solid with a dried glass stick. Alternatively, you can also use it the following. The bottle is put into a plastic bag and soaking in a water bath with warming at 40 degree C to dissolve the solid. Please be careful of volatilization of the organic solvent when the bottle is unsealing. |
|
Precautions |
It reacts violently with water. Air & moisture sensitive. Incompatible with oxidizing agents and bases. |
|
General Description |
Based on the provided literature, Benzylmagnesium chloride (or benzylmagnesiumchloride) is a Grignard reagent used in organic synthesis, particularly in reactions involving carbohydrate aldehydes, where it can lead to unexpected rearrangements (e.g., benzyl to o-tolyl carbinols). It also serves as an alkylating agent in the synthesis of high-valent metal complexes, such as tribenzyl derivatives of niobium and tantalum, which are further modified for catalytic applications like polymerization. Additionally, it is employed in the preparation of chiral phosphinate ligands for asymmetric hydrogenation reactions. Its reactivity is influenced by reaction conditions, including temperature, solvent, and reactant ratios. **Returned paragraph:** Benzylmagnesium chloride is a versatile Grignard reagent used in organic synthesis, including rearrangements of carbohydrate aldehydes (e.g., benzyl to o-tolyl carbinols), alkylation in metal complex formation (e.g., niobium/tantalum tribenzyl derivatives), and preparation of chiral ligands for asymmetric hydrogenation. Its reactivity depends on conditions such as solvent, temperature, and stoichiometry. |
|
Application |
Benzylmagnesium chloride (Grignard reagent) can be used as an alkylating reagent:For alkylating quinolyl-functionalized Cp?chromium(III) complexes which are used as precursors for the preparation of active olefin polymerization catalysts.In the synthesis of 2,3-disubstituted benzopyran-4-ones, 6-chloro-8-methylpurine derivative, and phenylalanine derivatives. |
InChI:InChI=1/C7H7.ClH.Mg/c1-7-5-3-2-4-6-7;;/h2-6H,1H2;1H;/q;;+1/p-1/rC7H7Mg.ClH/c8-6-7-4-2-1-3-5-7;/h1-5H,6H2;1H/q+1;/p-1
Analysis of a monoclinic modification of...
A polymer supported 'magnesium(anthracen...
Magnesium organometallic reagents occupy...
The continuous synthesis of Grignard rea...
The reaction of digallane (dpp-bian)Ga?G...
Grignard reagents undergo facile regiose...
benzyl chloride

2-methylanthracene

1,1'-(1,2-ethanediyl)bisbenzene

toluene

benzylmagnesium chloride
| Conditions | Yield |
|---|---|
|
With magnesium 9,10-dimethylanthracene * 3 THF; In tetrahydrofuran; for 3h; Yield given. Yields of byproduct given;
|
benzyl chloride

1,1'-(1,2-ethanediyl)bisbenzene

benzylmagnesium chloride
| Conditions | Yield |
|---|---|
|
With magnesium; In tetrahydrofuran; 2-methyltetrahydrofuran; at 60 ℃; under 5171.62 Torr; Solvent; Temperature; Inert atmosphere; Flow reactor;
|
83% |
benzyl chloride
(3-Methyl-2-phenyl-3,6-dihydro-2H-selenopyran-2-yl)-phenyl-methanone
9,10-dihydro-9,10-anthracendiyl-tris(THF)magnesium
diethyl ether
3-Phenyl-1-propanol
4-phenyl-butan-1-ol
1-benzyl-2-methylhydrazine
2-phenyl-1-(pyridin-4-yl)ethanol