Your Location:Home > Products > Organic Chemistry > Vinylmagnesium chloride
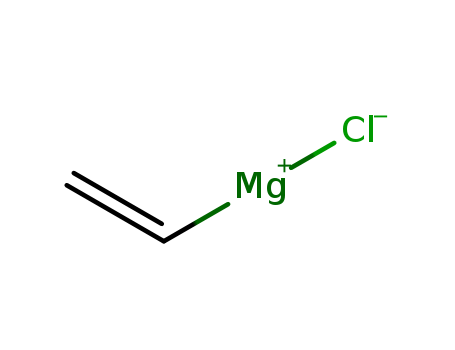
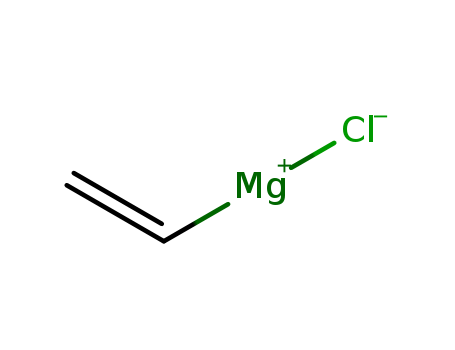
CasNo: 3536-96-7
MF: C2H3ClMg
Appearance: dark brown solution
|
Chemical Properties |
dark brown solution |
| Description | Vinylmagnesium chloride belongs to the family of Grignard reagents, which are organomagnesium compounds widely utilized in organic synthesis. Vinylmagnesium chloride is produced through a Grignard reaction, a common method for creating carbon-carbon bonds, involving the reaction of vinyl chloride (CH2=CHCl) with magnesium metal. |
|
Uses |
Vinylmagnesium chloride is a valuable Grignard reagent used to introduce vinyl groups into organic compounds, enabling the synthesis of more complex molecules. Its high reactivity and versatility make it an essential tool in organic chemistry, but proper handling and safety measures are crucial due to its sensitivity to air and moisture. |
InChI:InChI=1/C2H3.ClH.Mg/c1-2;;/h1H,2H2;1H;/q;;+1/p-1/rC2H3Mg.ClH/c1-2-3;/h2H,1H2;1H/q+1;/p-1
The invention relates to a synthesis met...
The invention relates to a phosphonium b...
chloroethylene

vinylmagnesium chloride
| Conditions | Yield |
|---|---|
|
With magnesium; In tetrahydrofuran;
|
|
|
With magnesium; In tetrahydrofuran; at 50 ℃; for 0.75h;
|
|
|
With magnesium; ethylene dibromide; In tetrahydrofuran; at 25 - 60 ℃;
|
|
|
With magnesium; In tert-butyl methyl ether; at 50 ℃; for 8h; Inert atmosphere;
|

chloroethylene

magnesium


vinylmagnesium chloride
| Conditions | Yield |
|---|---|
|
In tetrahydrofuran;
|
|
|
magnesium; With iodine; ethylene dibromide; In tetrahydrofuran; at 60 - 64 ℃;
chloroethylene; In tetrahydrofuran; at 60 - 64 ℃;
|

chloroethylene
magnesium
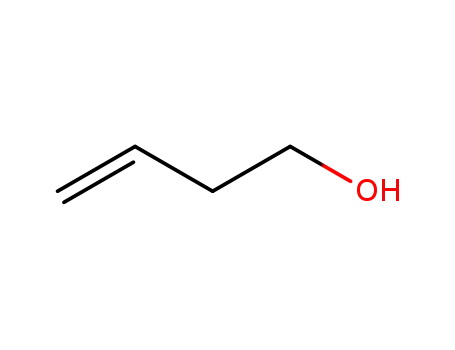
homoalylic alcohol
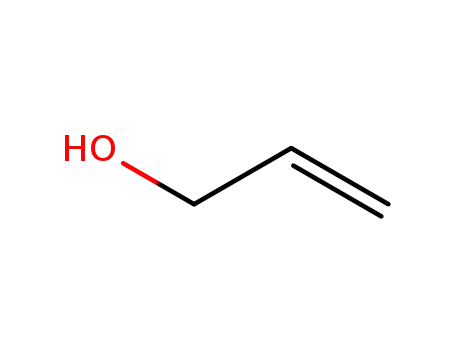
allyl alcohol

methyltrivinylsilane
Dichloromethylvinylsilane