Your Location:Home > Products > Organic Chemistry > Allylmagnesium chloride
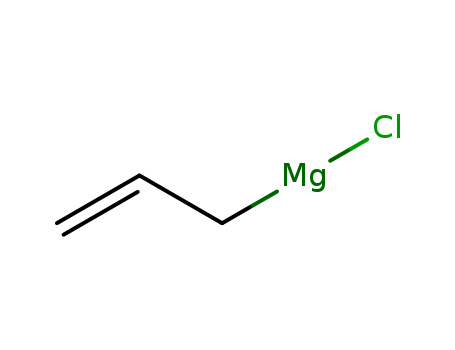

CasNo: 2622-05-1
MF: C3H5ClMg
Appearance: clear tan to brown, amber, dark grey, dark green
|
Chemical Properties |
clear tan to brown, amber, dark grey, dark green |
|
Uses |
Allylmagnesium chloride has been used in a study of the nucleophilic ring-opening of 2-methyleneaziridines to imines and subsequent conversion to 5,5′-disubstituted hydantoins. It is also used as a reagent for the introduction of the allyl group.Grignard-reactions: reagent for the introduction of the allyl group. |
|
Application |
Used in a study of the nucleophilic ring-opening of 2-methyleneaziridines to imines and subsequent conversion to 5,5′-disubstituted hydantoins. |
|
Precautions |
It reacts violently with water.Air & moisture sensitive. Stable under recommended storage conditions. |
InChI:InChI=1/C3H5.ClH.Mg/c1-3-2;;/h3H,1-2H2;1H;/q;;+1/p-1/rC3H5Mg.ClH/c1-2-3-4;/h2H,1,3H2;1H/q+1;/p-1
β-Trifluoromethyl vinamidinium salt 1 re...
The continuous synthesis of Grignard rea...
The invention relates to a phosphonium b...
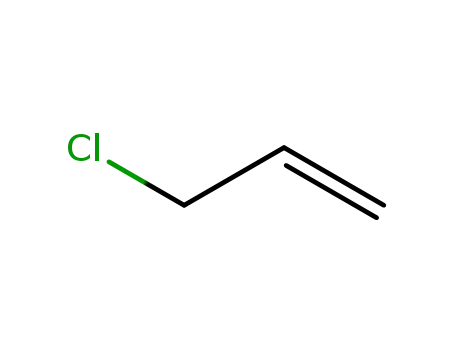
3-chloroprop-1-ene

propene

1,5-Hexadien

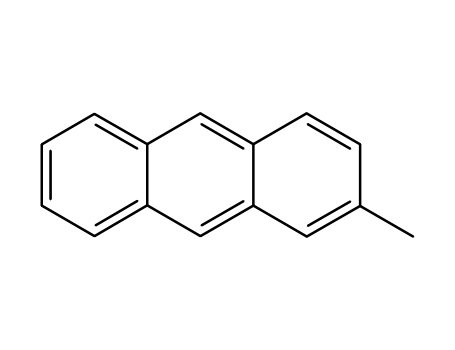
2-methylanthracene

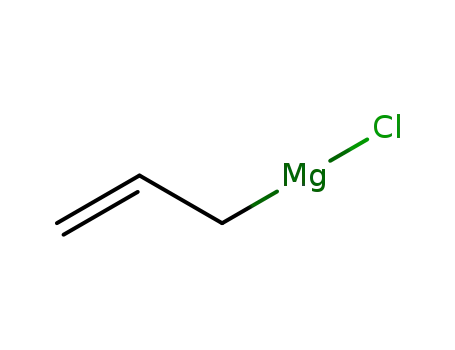
allylmagnesium bromide
| Conditions | Yield |
|---|---|
|
With magnesium 2-methylanthracene * 3 THF; In tetrahydrofuran; for 27h; Yield given. Yields of byproduct given; Ambient temperature;
|
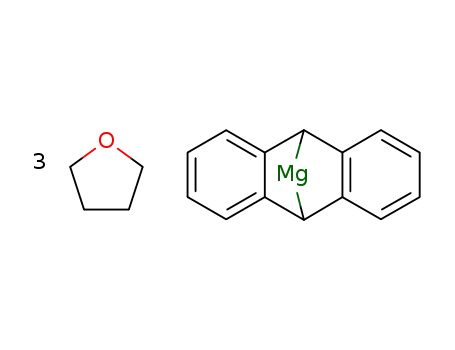
9,10-dihydro-9,10-anthracendiyl-tris(THF)magnesium

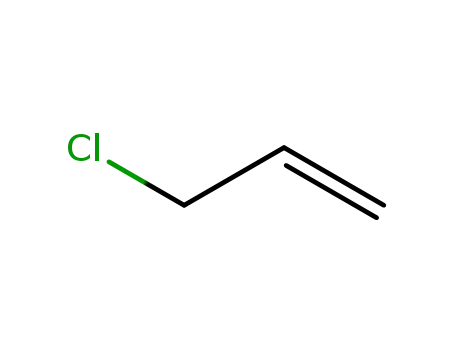
3-chloroprop-1-ene

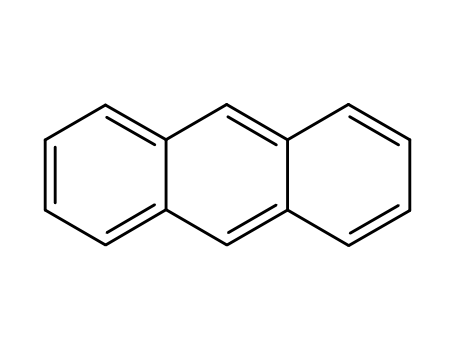
anthracene

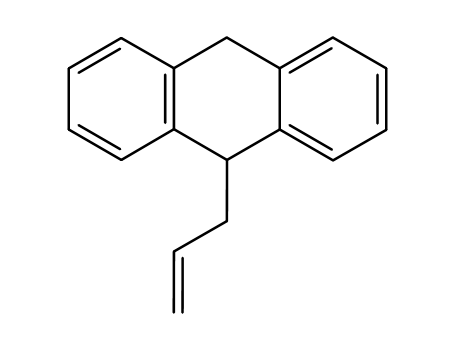
9-(2-propenyl)-9,10-dihydroanthracene


allylmagnesium bromide

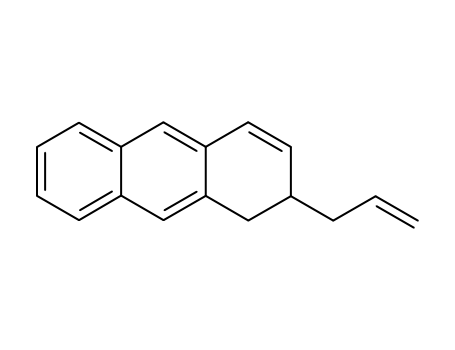
2-Allyl-1,2-dihydro-anthracene
| Conditions | Yield |
|---|---|
|
In tetrahydrofuran; at -78 ℃; for 12h; Further byproducts given. Yields of byproduct given. Title compound not separated from byproducts;
|

9,10-dihydro-9,10-anthracendiyl-tris(THF)magnesium
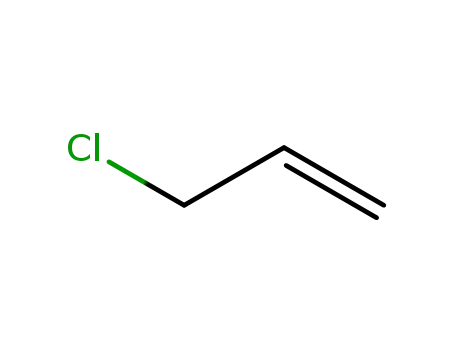
3-chloroprop-1-ene
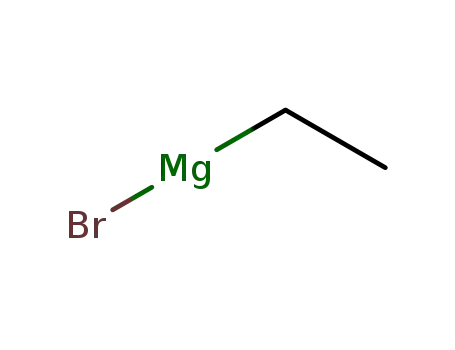
ethylmagnesium bromide
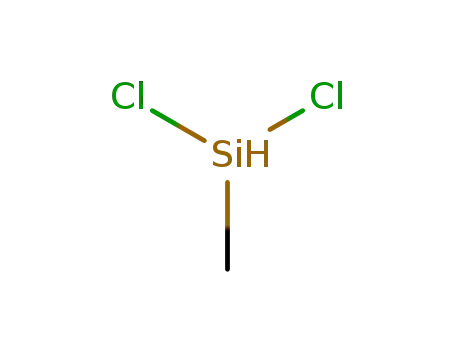
Dichloromethylsilane
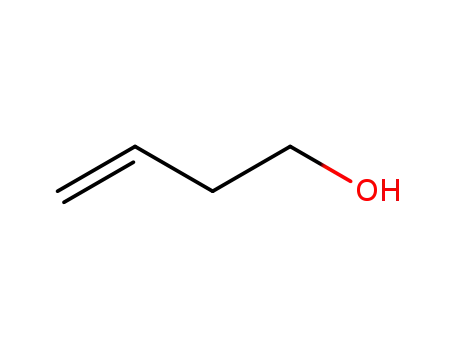
homoalylic alcohol
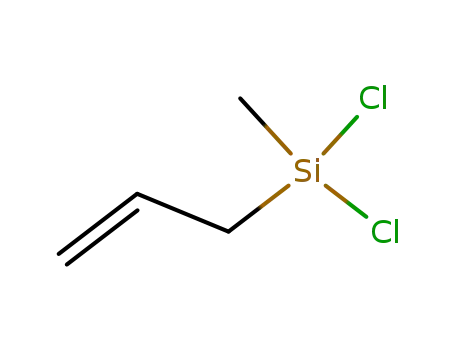
allyldichloromethylsilane
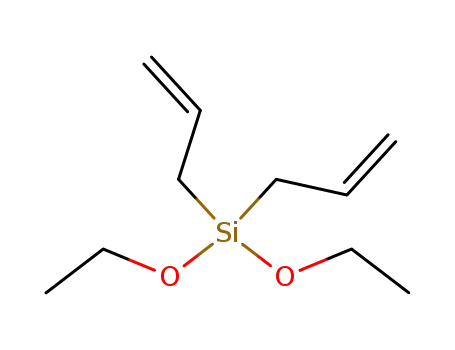
di(2-propenyl)diethoxysilane
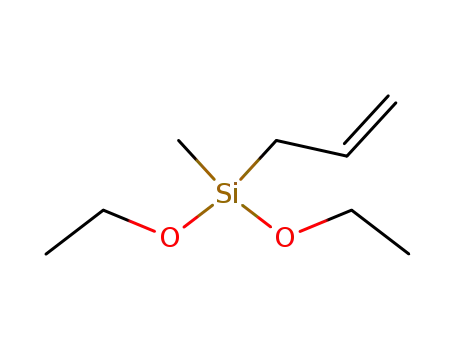
3-diethoxymethylsilyl-1-propene