Your Location:Home > Products > Organic Chemistry > Benzylmagnesium chloride
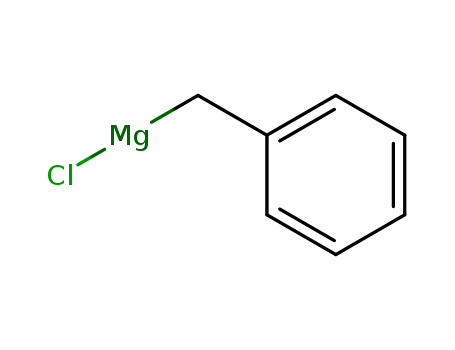


CasNo: 6921-34-2
MF: C7H7ClMg
Appearance: clear green-brown to brown-purple solution
|
Chemical Properties |
Light gray to dark amber-grey slurry or solution |
|
General Description |
Benzylmagnesium chloride, like other Grignard reagents, is sensitive to air and moisture. It serves as a Grignard reagent. |
|
Application |
Benzylmagnesium chloride is employed as a Grignard reagent, allowing for the addition of the benzyl group (C6H5CH2-) to organic compounds. This enables the creation of more complex molecules with altered chemical properties. It is used as an alkylating reagent in various chemical reactions. |
InChI:InChI=1/C7H7.ClH.Mg/c1-7-5-3-2-4-6-7;;/h2-6H,1H2;1H;/q;;+1/p-1/rC7H7Mg.ClH/c8-6-7-4-2-1-3-5-7;/h1-5H,6H2;1H/q+1;/p-1
Analysis of a monoclinic modification of...
The reaction of benzylmagnesium chloride in THF with monomeric formaldehyde has been been studied in detail.
Studies of the behavior of benzylmagnesium chloride toward a variety of reactants2,3 have shown thato-tolyl derivatives appear frequently among the products of the reaction.
Grignard reagents undergo facile regiose...
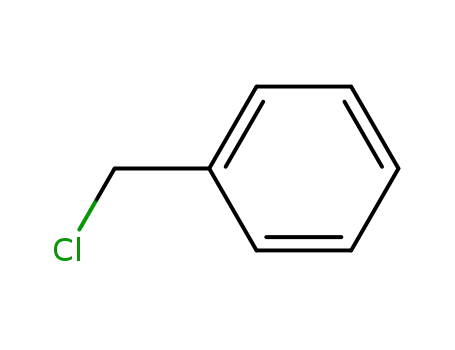
benzyl chloride

2-methylanthracene

1,1'-(1,2-ethanediyl)bisbenzene

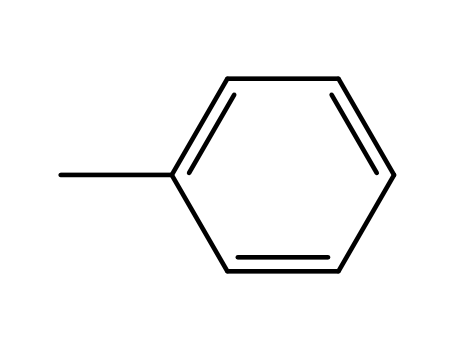
toluene

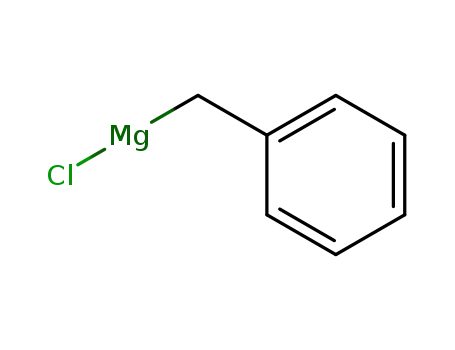
benzylmagnesium chloride
| Conditions | Yield |
|---|---|
|
With magnesium 9,10-dimethylanthracene * 3 THF; In tetrahydrofuran; for 3h; Yield given. Yields of byproduct given;
|
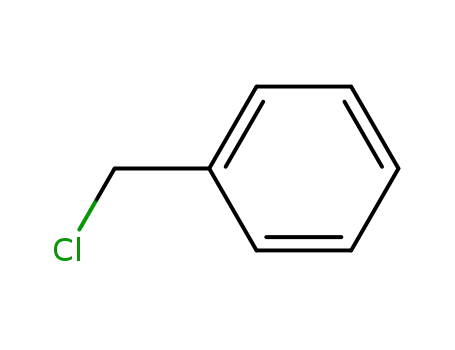
benzyl chloride


1,1'-(1,2-ethanediyl)bisbenzene

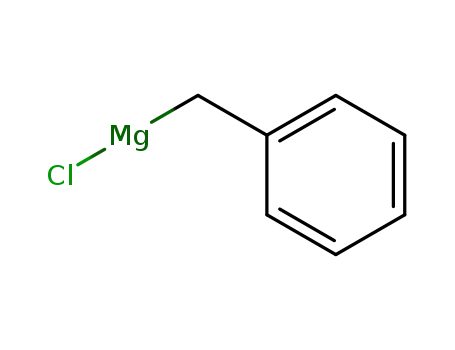
benzylmagnesium chloride
| Conditions | Yield |
|---|---|
|
With magnesium; In tetrahydrofuran; 2-methyltetrahydrofuran; at 60 ℃; under 5171.62 Torr; Solvent; Temperature; Inert atmosphere; Flow reactor;
|
83% |
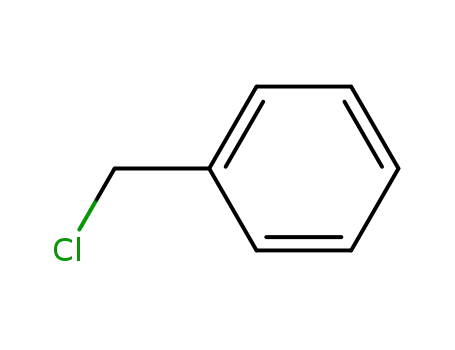
benzyl chloride
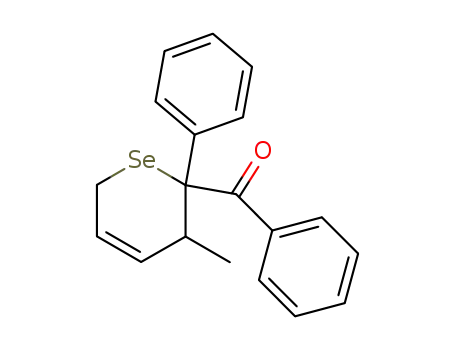
(3-Methyl-2-phenyl-3,6-dihydro-2H-selenopyran-2-yl)-phenyl-methanone
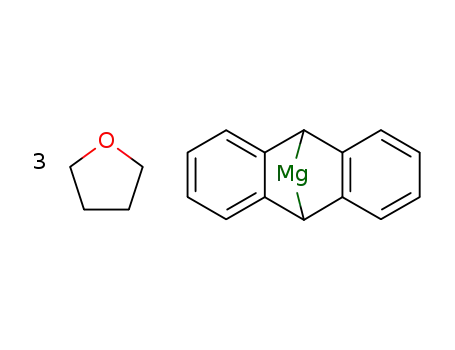
9,10-dihydro-9,10-anthracendiyl-tris(THF)magnesium
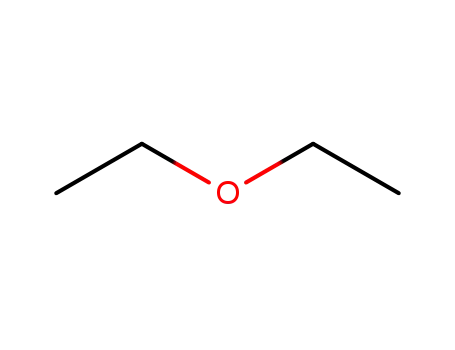
diethyl ether
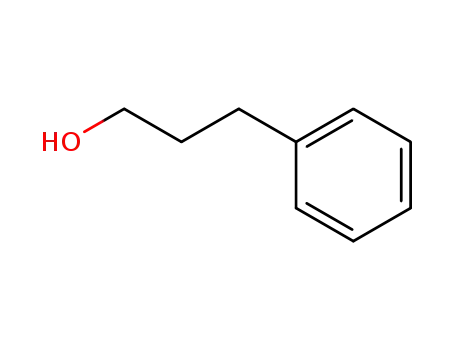
3-Phenyl-1-propanol
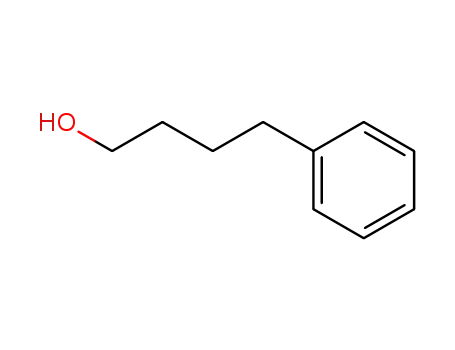
4-phenyl-butan-1-ol
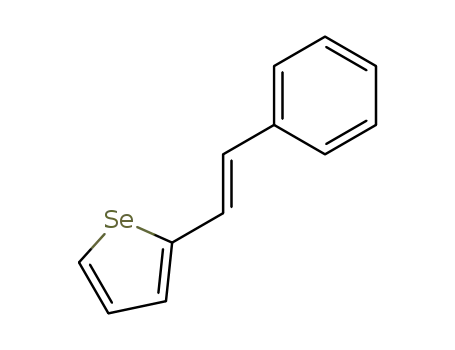
(E)-2-styrylselenophene
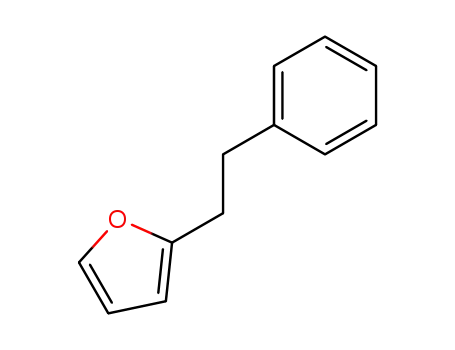
2-(2-phenylethyl)furan